FDA approves Tavneos for vasculitis – ChemoCentryx
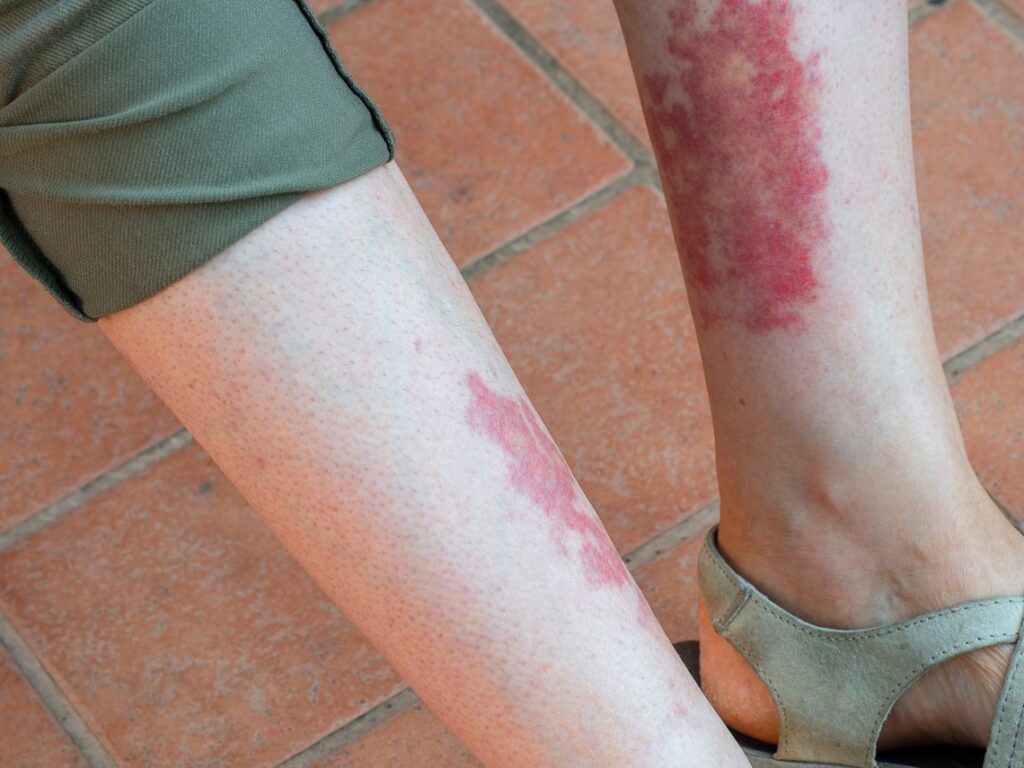
ChemoCentryx announced that the FDA has approved Tavneos (avacopan), an orally administered selective complement 5a receptor inhibitor, as an adjunctive treatment of adult patients with severe active anti-neutrophil cytoplasmic autoantibody-associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis), specifically granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) (the two main forms of ANCA vasculitis), in combination with standard therapy.
The approval in ANCA-associated vasculitis was supported by the results of the pivotal Phase III ADVOCATE trial, which were highlighted in the February 2021 edition of The New England Journal of Medicine (NEJM). The ADVOCATE trial of Tavneos was a global, randomized, double-blind, active-controlled, double-dummy Phase III trial of 330 patients with ANCA-associated vasculitis in 20 countries. Eligible study subjects were randomized to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either Tavneos or study-supplied oral prednisone. Subjects in both treatment groups could also receive non-protocol glucocorticoids if needed.
The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score, or BVAS. The study demonstrated superiority to a prednisone-based standard of care with respect to sustained remission at 52 weeks. The most common adverse reactions (at least 5% of patients and higher in the Tavneos group vs. prednisone group) were: nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.